The Laboratory has established, documented, implemented, and maintains a Quality System and ensures continuous improvement of its effectiveness in accordance with the requirements of ISO 15189:2012.
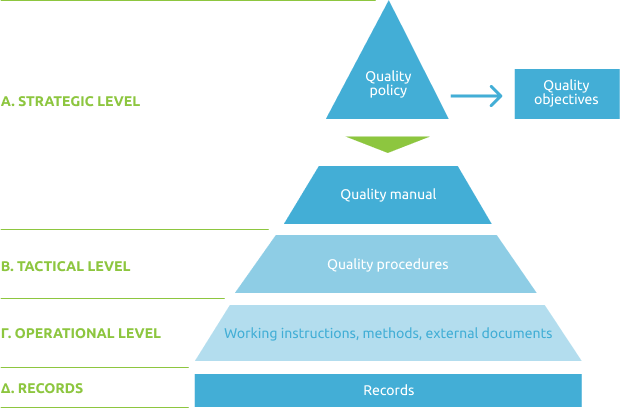
The Quality Management System is structured in four levels:
Each sample is fully traced from the moment of receiving it and at all stages of processing within the Istomedica Anatomic Pathology Laboratory.
- Each specimen, from the moment of its arrival at the Laboratory, receives a unique protocol number and a barcode. The sample is then processed, and the individual carrying out the procedure (technologist, pathologist, pathology anatomist, registrar) records each stage of processing in detail in the electronic case file
The quality of all tests performed, and services provided at Istomedica is ensured by:
- Conducting monthly internal inspections (temperature and cleanliness of premises and machinery)
- Conducting vertical inspections daily, where the correct implementation of the quality system is checked (intra-laboratory consultation, review of intra-operative consultation – frozen section randomized review of histological cases and evaluation of the quality of stains). Through vertical reviews, re-diagnosis of already completed cases is carried out for a second time, thus checking the repeatability and reproducibility of the findings by a different pathologist from the one who made the initial diagnosis
- Conducting horizontal inspections to check the correct application of the Procedures, Guidelines and testing protocols established, as well as the correct administrative and laboratory operation
In addition, all accredited examinations are subject to systematic inter-laboratory audits by the College of American Pathologists (CAP) on an annual basis. This process ensures the high level of service provided regarding the results quality assurance.
Record keeping
The sample remains within the Laboratory premises for a period of approximately one (1) month. Retention of the specimen for a longer period is possible only upon request (on the referral form) of the referring physician.
Paraffin cubes and slides are kept on file in the Laboratory for 10 years.
The data in the information system is automatically backed up daily. A data recovery test is carried out once every six months under the responsibility of the laboratory's external partner to check the effectiveness of the back-up carried out.
Green policy
ISTOMEDICA, with a focus to protecting human life, the company's employees, and the environment, carries out a series of actions within the framework of corporate social responsibility and green policy.
All toxic and contaminated waste is removed from the Laboratory by a qualified and certified provider, on a weekly basis, within special packing cells for burial and destruction. In particular:
| Waste category | Waste management method | |
|---|---|---|
| Urban type | Waste similar to household waste e.g., from food preparation in kitchens of health care establishments, from catering activities, glass, paper, cardboard, plastic, metals, packaging materials, and other non-hazardous materials | Landfill |
| Mixed Hazardous Waste | Tissues, toxic waste/reagents | Incineration |
| Sharp objects | Knives, blades, scalpels, broken glass | Incineration |
| Haxzardous Liquid Waste | Staining waste | Incineration |
For the processing of samples, substitute materials are used instead of alcohol and xylene, which do not harm the environment. In addition, the ventilation pipe system for volatile liquids inside the laboratory is routed to the external environment. The volatile liquids first pass-through activated carbon filters that capture the health-harmful substances before they are discharged into the environment. These filters are changed at regular intervals.
The small amount of xylene used in some processes is recycled by means of a special machine and reused to reduce the environmental impact of the volume of toxic waste.
We participate in the recycling of paper and plastic of the Municipality of Athens, while the air conditioners used for heating and cooling are equipped with the latest inverter technology.
Code of Conduct & Ethics
ISTOMEDICA has established and maintains rules of ethics and conduct which are recognized, supported, and adhered to by the management and staff of the laboratory. More specifically:
- The laboratory places the interests and satisfaction of the users of its services as its top priority
- The laboratory is organised to ensure that its management and staff are free from any internal and external commercial, financial or other pressures and influences which might adversely affect the quality of its work
- All users of the laboratory's services are treated equally and without discrimination on the basis of their sex, race or economic status
- Patient information is collected in a manner that ensures confidentiality and is limited to the information necessary for the performance of tests, the proper evaluation of results and the safety of staff
- Each employee is committed to ensuring the confidentiality of the information handled
- Each employee is committed to ensuring that there is no conflict of interest, while aiming at safeguarding the rights of users of the laboratory's services
- The relationship between the laboratory and medical practitioners is based solely on the interests of patients and follows the rules of internationally accepted scientific ethics
- The laboratory ensures the reliability of the tests it carries out. Tests are carried out in accordance with internationally accepted methods, by personnel with the necessary competence and experience and the results obtained shall not be falsified or altered in any way
- If a sample is received from the laboratory in a condition which renders it totally unsuitable for the requested test, it is rejected, and the appropriate authority is notified
- The laboratory ensures that the medical record is stored in a manner that protects against loss, unauthorised access, falsification, or other misuse and in accordance with applicable laws and guidelines
- The use of samples or laboratory results for research/statistical purposes is only permitted if these are handled anonymously